
Developing a standardized format for accompanying documentation and a mechanism for data transmission or retrieval is being designed to eliminate the need for individual solutions between various industry stakeholders. By standardizing these processes, transparency is increased, and efficiency along the supply chain is enhanced.
Accompanying documents such as order confirmations, delivery slips and invoices will be provided exclusively in electronic form and in a standardized format to goods recipients—ranging from pharmacies and hospitals to manufacturing companies and pharmaceutical wholesalers. In doing so, established concepts, such as the Forum Elektronische Rechnung Deutschland (FeRD), will be utilized.
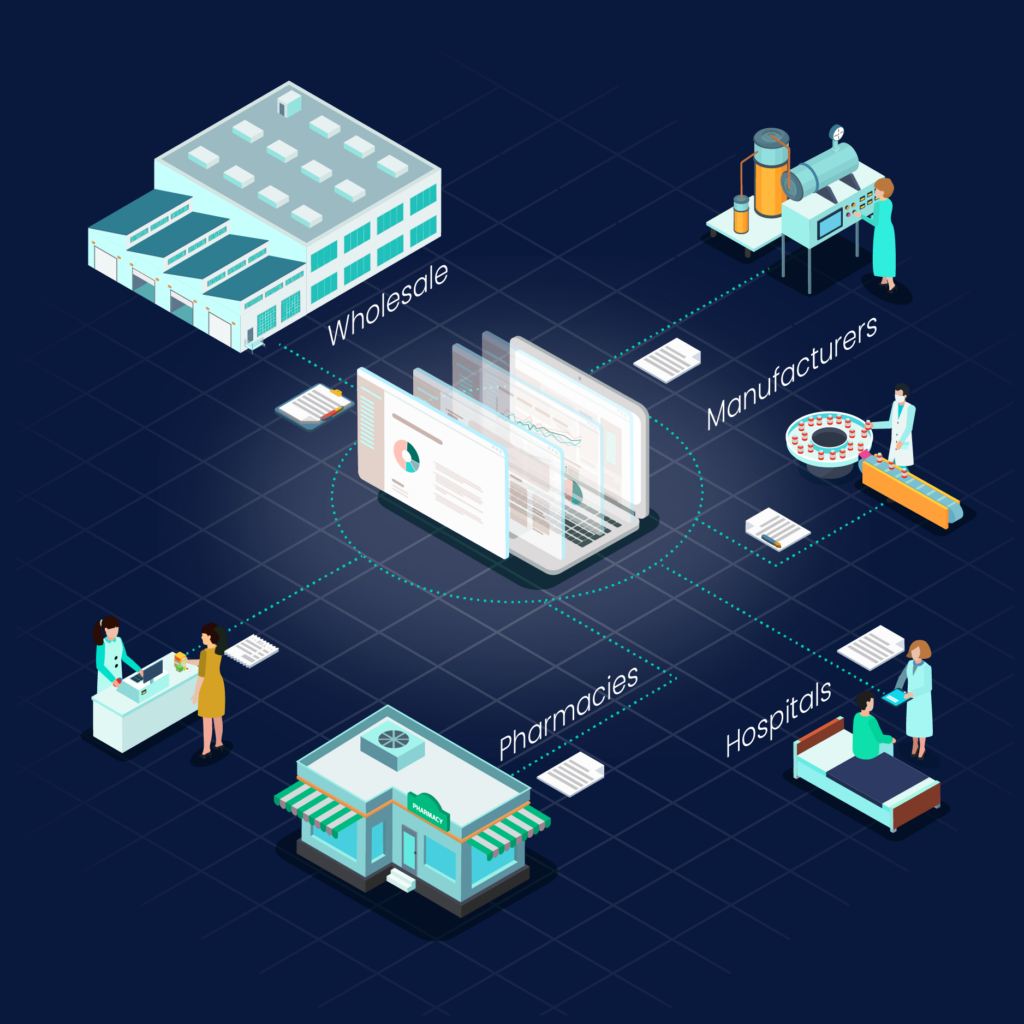
All order confirmations, delivery slips, and invoices will be digital in a uniform data format.
All recipients of goods benefit—from pharmacies and hospitals to manufacturing companies and pharmaceutical wholesalers.
By standardizing processes, we increase transparency and boost efficiency in the supply chain.
Establishing this basic standard opens up further areas of application. In the future, accompanying data such as serial numbers, certificates, or information on dispatch notifications and return processing could also be transmitted. It is also conceivable to use push notifications to communicate product recalls. This holistic concept lays the foundation for a future-proof, digitally networked supply chain in the healthcare industry.
Would you like to become part of our idea or do you have a question?
Please send us a message using the contact form.
Design & technical implementation: powered by Gesundheitsforen
Status of the information: September 2024